Clinical Data Abstraction
Atlas is a web-based comprehensive abstraction software that collects data from the Electronic Medical Record (EMR) and transfers it into research databases using a combination of AI-assisted recommendations and clinical abstractors.
Product Type
B2B Data Abstraction
Primary User
Clinical Data Abstractor
Project Maturity
New product build to stabilization and growth
Overview
-
A clinical registry tracks information about the health status of patients and the care they receive for a specific disease or condition. Registries bring together data to be used in quality improvement initiatives, policy decisions, and to support research and treatment development. In this context, it is important for registries to maintain high standards of completeness and accuracy for the clinicians, researchers, and public who use the information.
In order for patient care organizations to participate in registries and gain insights into benchmarking, clinical effectiveness, and more; they must process their data and submit it to the registry governing body. Clinical data abstraction is the process of searching through medical records—electronic and/or paper—to identify the data required for secondary use. This process results in the summary of information about a patient encounter, referred to as “cases”. -
Clinical data abstraction is a challenging process due to a variety of factors inherent to the healthcare domain and the complexity of the data involved. Improving the clinical data abstraction process allows healthcare organizations to make the most of limited resources, including a shortage of skilled abstractors.
Opportunity: Data Variety & Volume
Healthcare organizations generate massive amounts of data daily, and making reviewing even a single patient’s data time-consuming. Clinical data comes in various formats, including textual notes, structured records, images, and more. Additionally, healthcare systems often use disparate electronic health record (EHR) systems, requiring abstractors to work with multiple systems to access the necessary data.
Seamlessly integrating multiple data sources into a single interface, and providing the user with tools to quickly identify relevant content in larger documents improves efficiency.Opportunity: Human Error & Time Constraints
The manual nature of data abstraction leaves room for human error, including misinterpretation, data entry mistakes, and omission of critical information. As a time-sensitive service, the additional pressure to complete tasks quickly can impact overall accuracy.
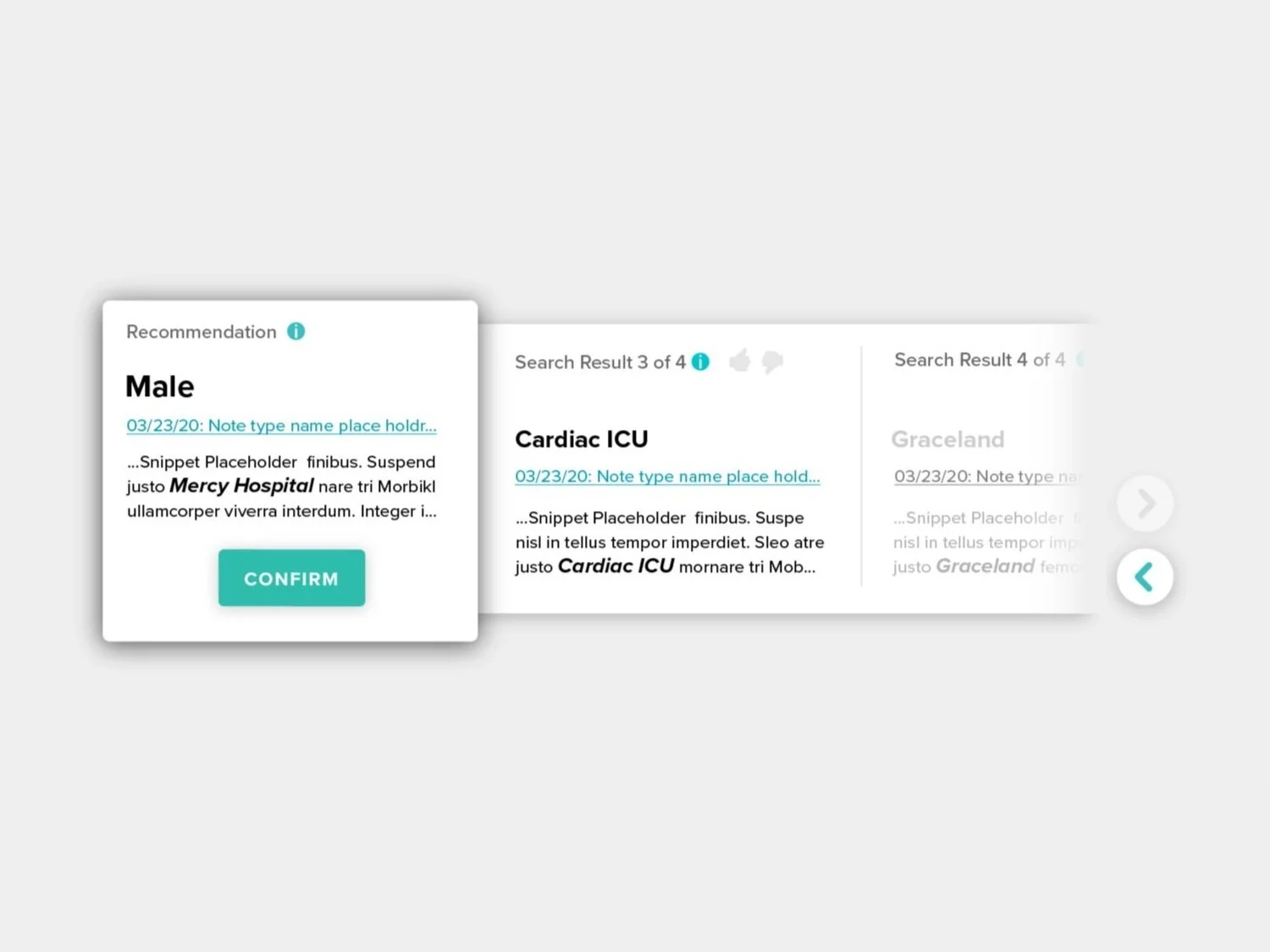
Automating structured data fields of a form, such as medication doses and date/time fields, reduces data entry errors and increases abstraction speed. NLP models can suggest recommendations for more complex fields, and display their sources material for abstractors to evaluate. -
For each individual project, a systematic and iterative approach is used to create a feature's structure, functionality, and user interface.
Requirements Analysis & Problem Definition:
The first step of any project is to gather and review requirements from stakeholders. Any gaps in the requirements are filled by additional research and user interviews. The purpose and scope of the project is then defined with functional and non-functional requirements, opportunities, and constraints such as budget, time, or technical limitations called out. This document gives a common point of reference throughout the project to keep the team aligned on the project scope.
Design:
A diagram of the feature architecture, noting the main components and user actions, identifies the design deliverables. Once complete, a quick sync with the product and development teams gives the opportunity to discuss any potential challenges uncovered and adjust time estimates for the project phases.Individual modules and components are designed or sourced from the design system, with visual and behavioral specifications noted. Designs are based on function, brand identity, and accessibility standards. Low or high-fidelity wireframes give additional clarity on feature placement, interactions, or responsive design expectations.
In some cases, functional prototypes validate design decisions and are used to gather feedback from stakeholders prior to development.
Review & Implementation:
Stakeholders participate in a final design review, where minor design adjustments are made based on feedback and modifications to requirements.The design hand-off package includes project requirements, architecture diagrams, wireframes/prototypes, and component specifications.
During the development and testing process, the design team is available to clarify any questions, and evaluate adjustment requests that may facilitate implementation. Any challenges or changes are be noted in the project document.
Maintenance & Feature Reviews:
After deployment, design performs regular evaluations and user feedback sessions to support feature refinement and evolving requirements. This may include reviewing common error feedback, unexpected slow-downs in user process, or conducting competitive analysis against similar market features.
Throughout the design process, communication and collaboration between the team and stakeholders is critical. The design process may vary depending on the project's complexity and specific requirements, but by having a detailed scope, regular feedback opportunities, and performing regular design reviews, we can be confident that we are producing a well-designed and reliable product. -
UX/UI design was part of the product team, which initially consisted of a head of product, a product manager, and myself as lead designer.
A common workflow was to receive high-level goals from leadership, which then required research and requirement definition in order to be integrated into the roadmap. Specific functional tasks could be brought forward by any team member (including cross-team), and were evaluated and prioritized collaboratively as needed. Tasks were managed through JIRA, with weekly sprints and team meetings to present deliverables, receive feedback, and discuss challenges.
Culturally, our team embraced challenges- placing high value on cross-team collaboration, continuous learning, personal integrity, and quality of deliverables. -
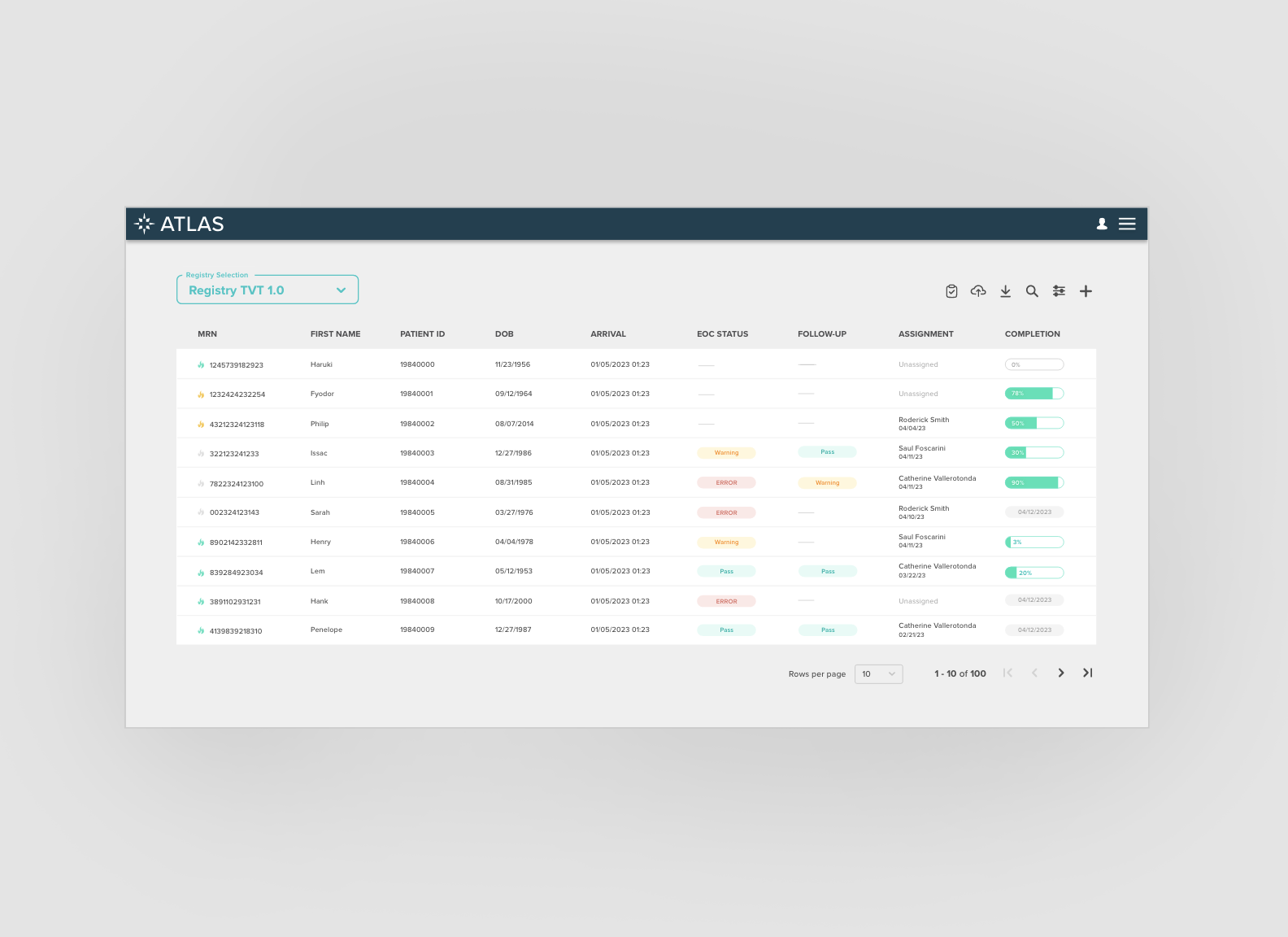
Validation: Interface summarizing data entry issues that impact case workflow, and providing internal communication tools. View validation case study
Submission Dashboard: Dashboard for internal users to track case submission status and identify failed case files. View submission history case study
Project Snapshots